In Unit V, we consider the speed, or rate, of chemical reactions. Thermodynamics tells us if a particular reaction will be spontaneous, but not how fast it will occur. Thus, both thermodynamics and kinetics are necessary for understanding chemical reactions. By the end of this unit, viewers should be able to analyze kinetics data to determine the order of the reaction with respect to any reagent, write simple reaction mechanisms, and determine if a mechanism is consistent with experimental data. They should be familiar with the steady-state approximation, rate-determining steps, and activation energy barriers. Viewers should be able to describe the properties of a catalyst and be familiar with terms associated with enzyme catalysis.

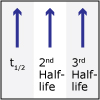
Lecture 30: Kinetics: Rate Laws
 Lecture 31 Notes" />
Lecture 31 Notes" />
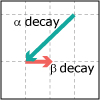
Lecture 31: Nuclear Chemistry and Chemical Kinetics
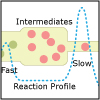
Lecture 32: Kinetics: Reaction Mechanisms
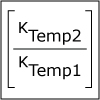
Lecture 33: Kinetics and Temperature
Lecture 34: Kinetics: Catalysts
Lecture 35: Applying Chemical Principles
Looking for something specific in this course? The Resource Index compiles links to most course resources in a single page.